An atom is made up of three constituent parts: electrons, protons, and neutrons. These parts of an atom are known as subatomic particles. Each of the three subatomic particles of an atom has a special associated charge: with electrons carrying a negative charge, protons having a positive charge, and neutrons having no net charge.
According to the Standard Model of Particle Physics, protons and neutrons constitute the nucleus of an atom while electrons revolve around the nucleus in a cloud. In an atom, the negatively charged electrons are attracted to the positively charged protons in the nucleus through the electromagnetic force. Electrons can be removed from their respective orbits by applying external force. However, electrons in orbits closer to the nucleus have stronger attractive force and require greater external force to be removed from their orbits.
Atoms with an equal number of electrons and protons are electrically neutral. While atoms with a deficient or more number of electrons than protons are known as ions. Atoms have the ability to transfer or share their farthest electrons with other atoms. Through this mechanism, atoms can bond into molecules and other chemical compounds.
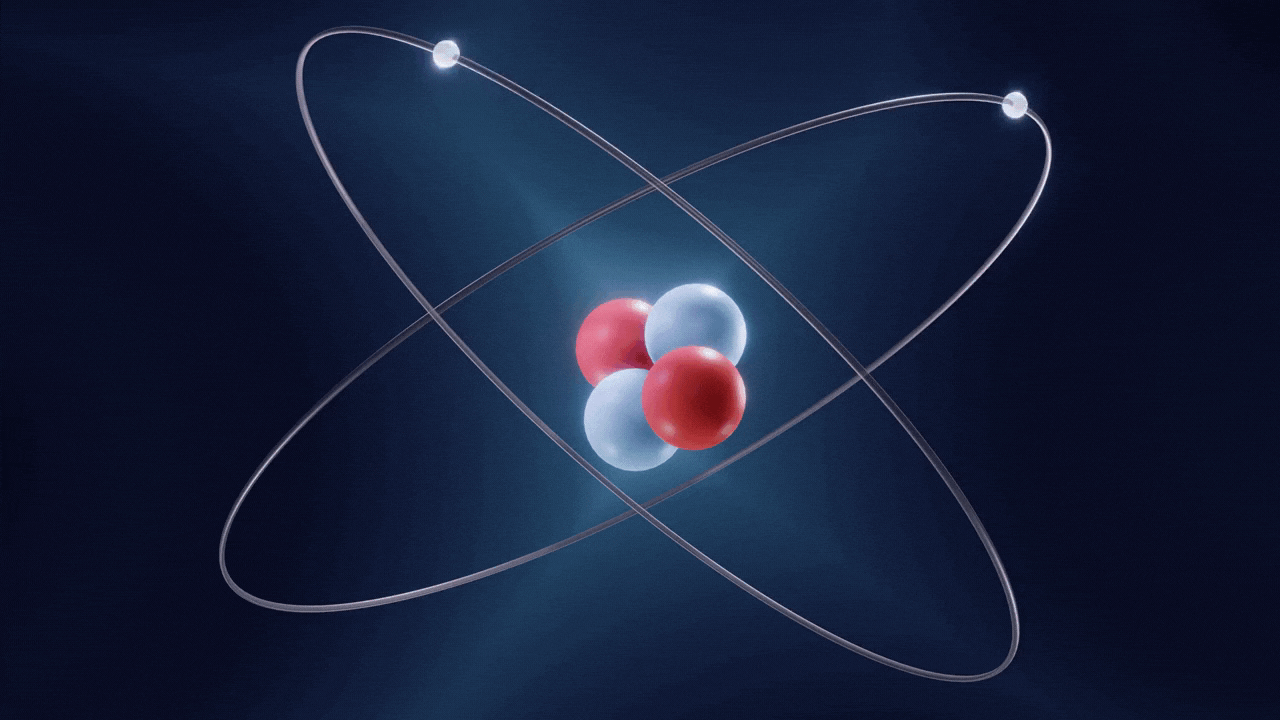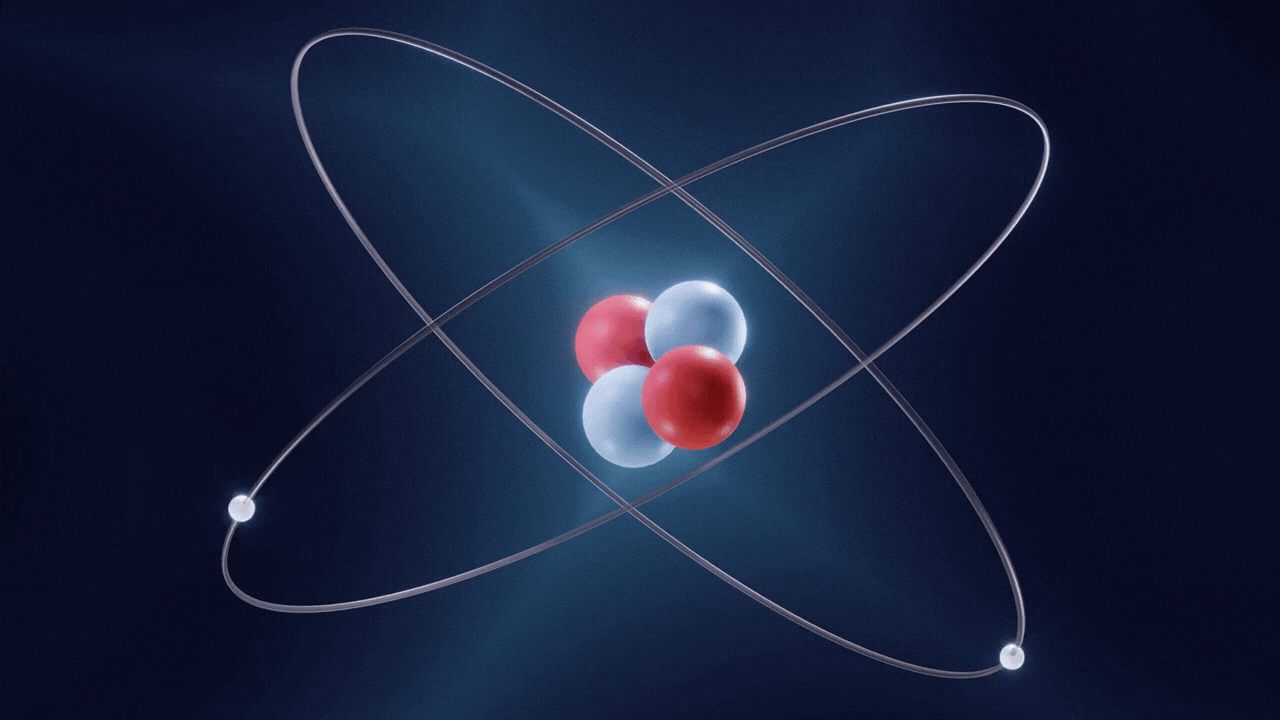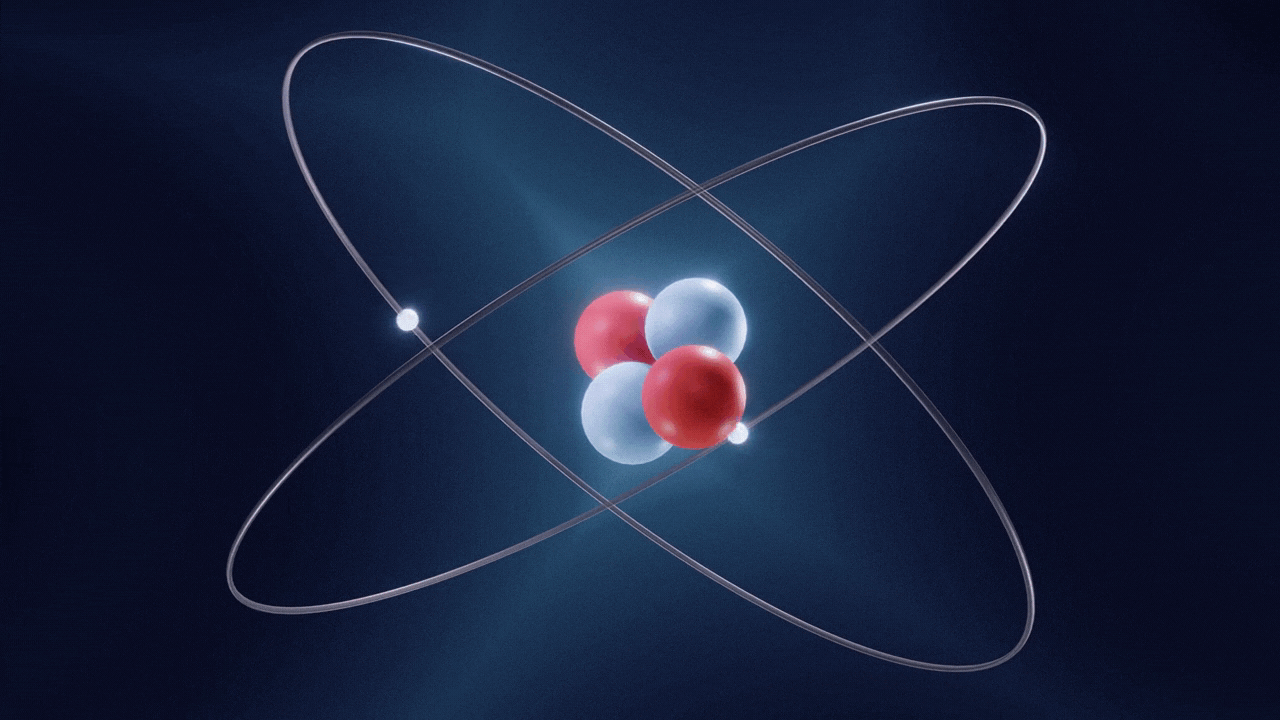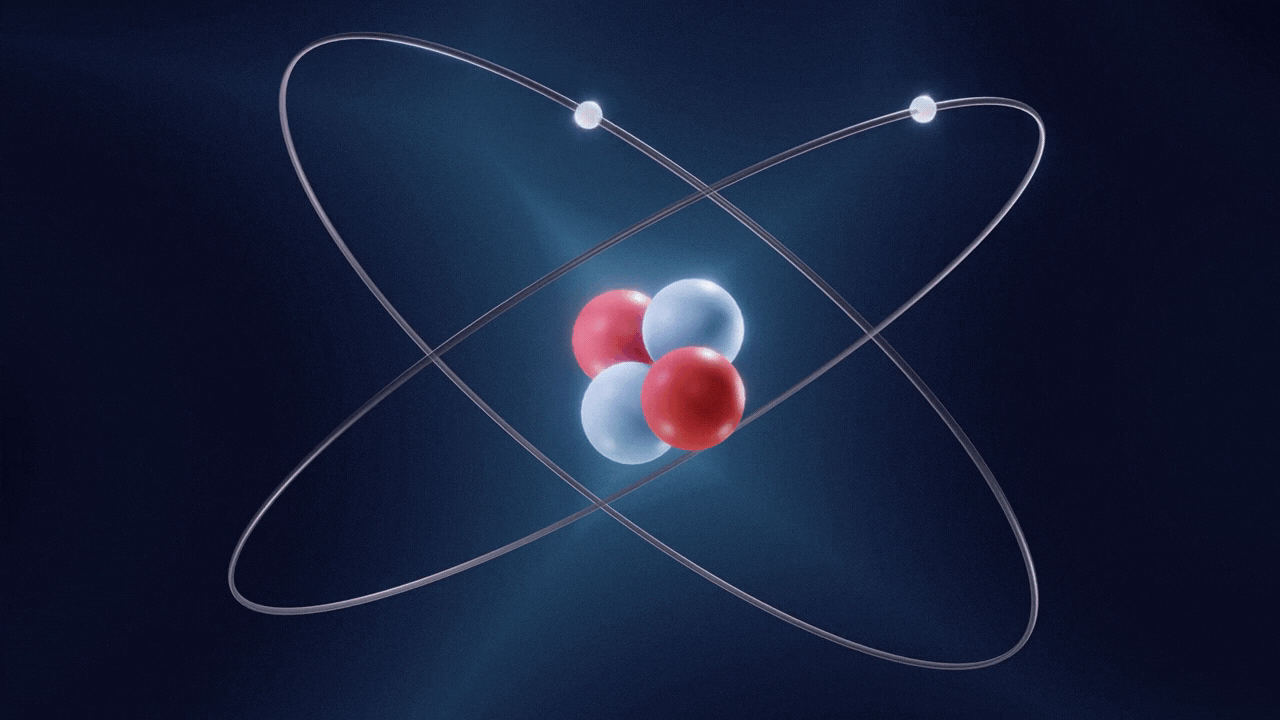
All the three subatomic particles of an atom are Fermions. A fermion means any particle of matter having an odd-half integer spin (such as ½, ⅔, etc). Fermions are either elementary or composite in nature. An electron is a type of the particles known as Leptons. Leptons are elementary in nature, which means they are not composed of other particles, do not undergo strong interactions, and have a half integer spin (½ spin). There are six types of Leptons that are called ‘flavours’; electron, muon, tauon, (these three are charged) and electron neutrino, muon neutrino, tau neutrino (these three are neutral).
While protons and neutrons are composite in nature, which means they are made up of other particles known as quarks.
Now let’s discuss all the three parts of atom individually:
ELECTRON
Electron is the smallest particle of an atom that is around 1,800 times smaller than a proton or neutron and has a negative charge.
Electrons revolve around the nucleus in multiple pathways known as orbits. Each orbit represents the particular energy level of electrons. When an electron absorbs a photon of sufficient energy, its energy level changes from lower to higher, and so it acquires a new quantum state. Similarly, when an electron releases excess energy as a photon, its energy level drops from higher to a lower level.
The electron configuration of an atom describes the location and distribution of electrons in its atomic orbitals. Chemists use the electron configuration and principles of physics to predict the properties of an atom, such as stability, conductivity, and boiling point.
PROTON
Protons are positively charged particles located in the nucleus of an atom. According to the Jefferson Lab, protons are around 99.86% as massive as neutrons.
The number of protons is unique in atoms of each element. The number of protons in the atoms of an element represents the atomic number of that element. For example, hydrogen atoms have one proton, carbon atoms have six protons, and oxygen atoms have eight protons. So, the atomic numbers of hydrogen, carbon, and oxygen are 1, 6, and 8 respectively.
A proton is made up of three quarks; two “up” quarks (each with a +⅔ charge) and one “down” quark (with – ⅓ charge), which calculate to a charge of +1.
NEUTRON
Neutrons are uncharged particles found in the atomic nuclei of all elements except hydrogen. A neutron is slightly more massive than that of a proton. Like protons, a neutron is also composed of quarks: one “up” quark (with +⅔ charge) and two “down” quarks (each with a – ½ charge), which works out to a 0 net charge.