What Is The Largest Molecule
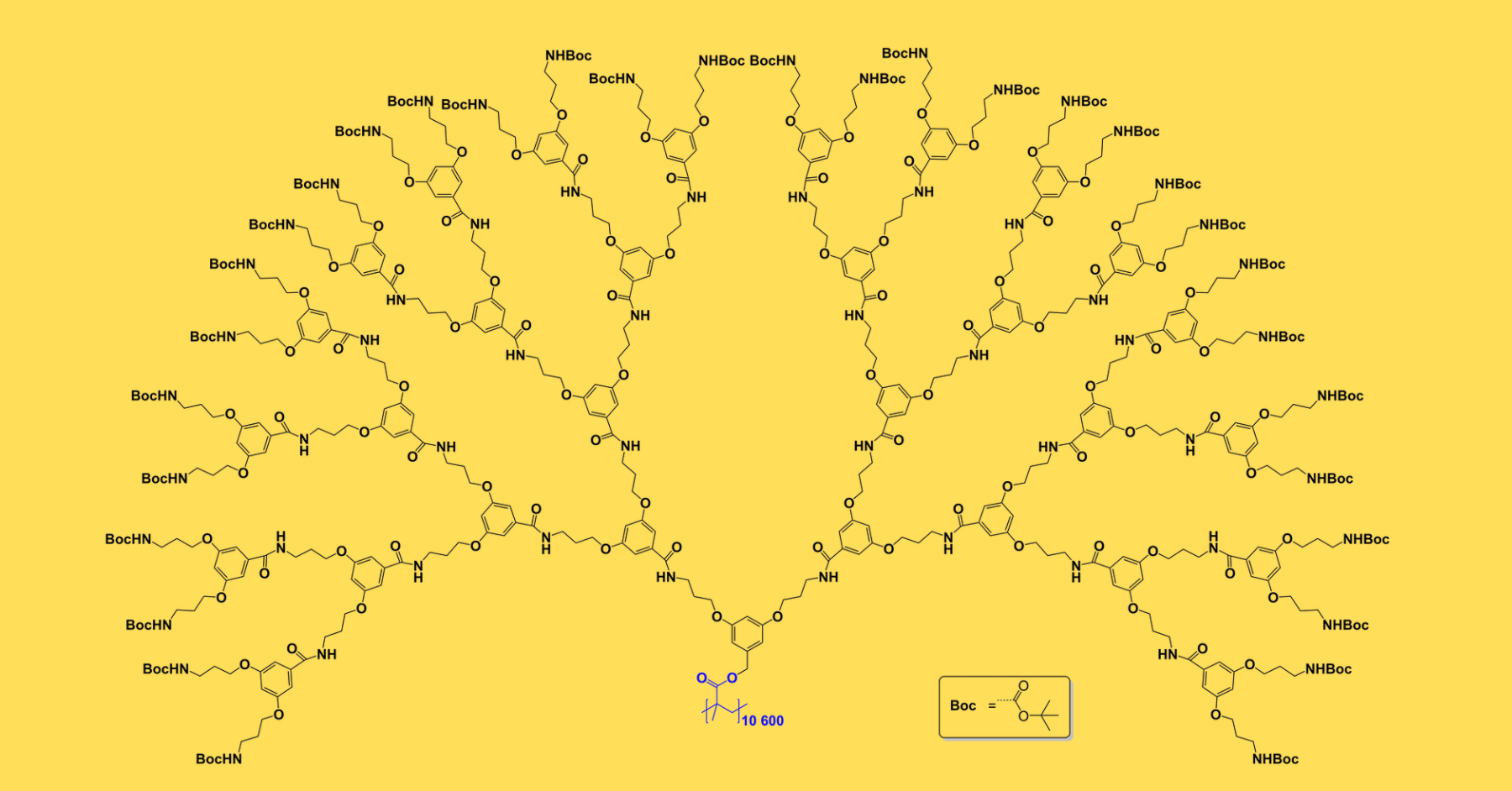
- The biggest stable molecule ever made is PG5.
- It is a synthetic molecule with a diameter of 10 nanometer. Its mass is 200 MDa, which is equal to 200 million hydrogen atoms.
- The PG5 molecule is a few micrometers long with a cylindrical shape that has 94.3% structure perfection.
- This molecule has about the same size as the tobacco mosaic virus, and has the ability to maintain its rod-like structure under various conditions.
- This molecule was made by Dieter Schlüter and his colleagues at the Swiss Federal Institute of Technology in Zürich.
- To build this giant molecule, Schlüter and his colleagues started with a standard reaction of polymerization, in which small molecules join together to form larger chains. They used a common carbon-hydrogen backbone, to which they added branches of benzene rings, nitrogen, carbon, and hydrogen. To attach groups of atoms to the backbone, they used reactions from the other sections of organic chemistry. They repeated the similar cycle again and again, adding sub-branches to every spreading branch. As a result, a tree-like structure was built, which they named PG5. A total of 170,000 bond formations were used in the whole synthesis.
- According to Schlüter, because of the use of standard reactions in the preparation of PG5, the work of his team should inspire other researchers to build synthetic macromolecules that they were formerly ‘not brave enough’ to try.
- Schlüter says that PG5-like large molecules could discover applications to store and deliver drugs. The drug could either dock to the surface of such molecules through different branches or settle in the spaces that are produced when the molecule folds in on itself. Schlüter says “There is not a single entity that can challenge the loading capacity of our PG5”.
🔬 Subscribe to SciMail
Get the latest science discoveries straight to your inbox!
Biggest Molecule In Space – Largest Molecule In Space
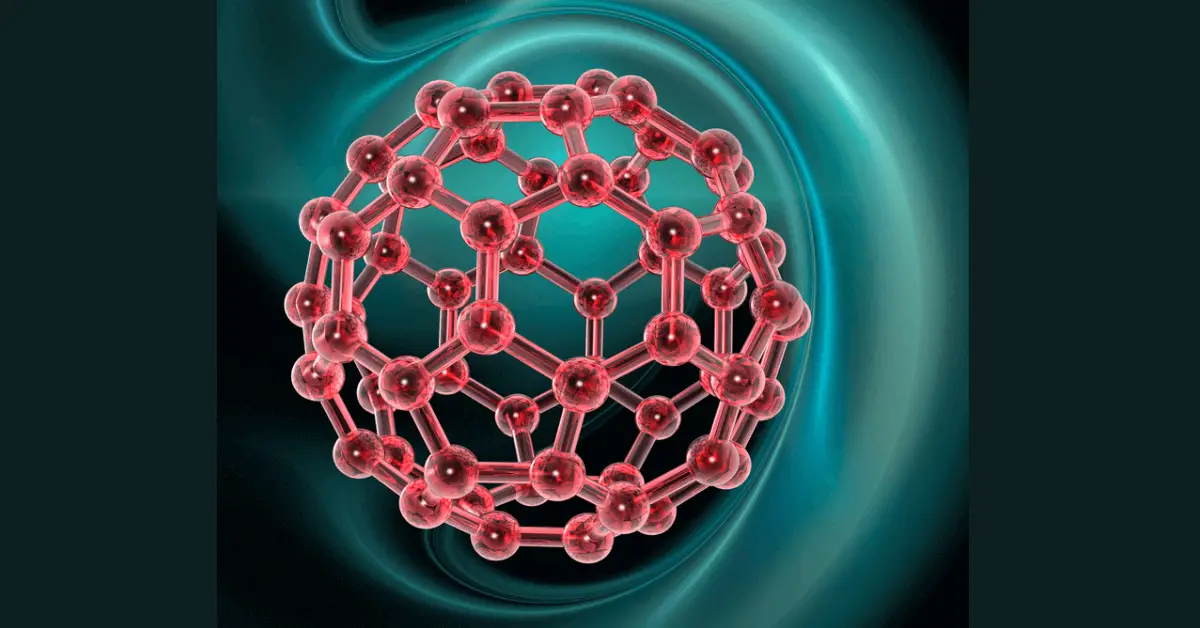
- Largest molecule in space is C60, which is made up of 60 carbon atoms.
- It was discovered in the nebula of a distant white dwarf star.
- This molecule is also called buckball or fullerene, because it resembles the geodesic domes of Bucky Fuller.
- The structure of this molecule is exactly like a black and white soccer ball. However, it is a microscopic scale molecule.
- Its size is around one nanometer, a human hair is approximately ten-thousand times thicker than this molecule.
- An astronomer at the University of Western Ontario and SETI Institute of California, Jan Cami and his team reported the discovery of this molecule in July 2010, in the journal Science.
- According to him, C60 is the biggest molecule ever found in space.
- They discovered it while they were examining light from the vicinity of a planetary nebula, which is a region of expanding gasses around an aging star about 6,500 light years away.
- They found these molecules accidentally while studying the planetary nebula Tc1 through NASA’s Spitzer infrared telescope.
- According to Cami, these buckyballs were initially identified in the 1980s in the laboratories on Earth. The discovery of these buckyballs triggered the entire field of nanotechnology because they have unique physical, chemical, and electrical properties. Since then, a completely new research field was built upon the properties of these molecules.
- Cami speculated that maybe the buckyballs have been in abundance on early Earth, and even they might have helped to start life on Earth because of their extreme complexity.
- He says that there are some C60 (buckyballs) that naturally exist on Earth. Scientists have later discovered them in the past few decades after their discovery in the laboratory setting. He says that very small quantities of these molecules were discovered in specific types of minerals that also naturally exist.
- However, now scientists realize that these molecules are abundantly found in space. Cami discovered so many molecules in space that if someone put it all together, it would have a similar mass as the moon.

Leave a Reply